By Shan Daniel, Smeralda Charles, and Mila Kocic in partnership with Climate Talks /Emory University.
Industrial Carbon Removal
Shan Daniel
Global warming poses a significant series of risks to humans on Earth. The planet is warming due to the greenhouse effect, or gases in the Earth’s atmosphere trapping longwave radiation emitted by the Earth’s surface within the atmosphere. The primary gas that is contributing to this effect is carbon dioxide, which is emitted during the combustion of hydrocarbon fuels for energy. The potential negative effects of unchecked emissions and warming result in extreme weather events, agricultural losses and flooding of coastal regions due to polar ice caps melting. As a result, switching global energy reliance to sources that mitigate the negative outcomes of climate change are critical and time-sensitive. This can be achieved by reducing global reliance on fossil fuels and other carbon-emitting substances and switching towards renewable energy, like solar, wind, hydroelectric, tidal, and nuclear, that do not release CO2 during their process (1).
However, since the start of the Industrial Revolution, atmospheric concentrations of CO2 have increased from 280 ppm to 430 ppm, equivalent to 1.18 × 10^12 additional metric tons of CO2 in our atmosphere. Given the magnitude of this change, removing emitted carbon from the atmosphere and storing it in a way that eliminates its effect on global warming has been a recent topic of interest for climate scientists.
Current carbon sequestration processes are tasked with taking air from the environment and concentrating it for storage (2). This can be achieved through a series of four reactions: Reaction 1 involves passing air through a solution of potassium hydroxide, where CO2 can react with water to become carbonic acid, which undergoes an acid base neutralization to generate potassium carbonate and water. Reaction 2 regenerates potassium hydroxide by reacting the potassium carbonate with calcium hydroxide to generate calcium carbonate, which is insoluble in water. In reaction 3, the solid calcium carbonate is subjected to thermal decomposition under extremely high temperatures to generate calcium oxide and carbon dioxide, which is emitted from the reaction in a controlled environment where it can be stored. Lastly, reaction 4 involves the calcium oxide regenerating calcium hydroxide when dissolved in water. Although reactions 1, 2 and 4 are exergonic, favorable reactions, the thermal decomposition of calcium carbonate is hugely endothermic and requires an extreme amount of energy input, making industrial carbon sequestration expensive and difficult to sustain (3). Per metric ton of CO2 removed from ambient air, the cost is estimated to be between $600-$1000. Using solely current carbon sequestration technologies, the cost of removing enough CO2 to return to pre-industrial levels of atmospheric CO2 would be $708 trillion, or roughly 25 times the US GDP in 2023 (4).
This methodology is not currently a viable method to meaningfully impact the levels of CO2 in our atmosphere. Innovation and improvement on both the third reaction of the carbon sequestration process and the storage/sales of the produced CO2 can reduce the overall cost of this process, allowing for its implementation on a larger scale and an overall larger positive impact (4). Capture of methane, a super pollutant which harms health and drives climate change, can complement carbon sequestration strategies by targeting short-lived climate pollutants that offer immediate health and climate benefits. However, with further innovation to create a more cost-efficient process and combination with other renewable forms of energy, carbon sequestration can be an important tool for mitigating the negative effects of climate change.
Regenerative Agriculture: Carbon Sequestration Using Biological Processes
Smeralda Charles
Regenerative agriculture (RA) — image from https://www.facebook.com/photo/?fbid=979398277567929 .
A biologically-driven method that has gained increasing recognition in recent years is Regenerative Agriculture (RA). Regenerative Agriculture offers a more integrated and sustainable approach to farming by focusing on soil restoration and long-term ecological balance (5). Unlike conventional agriculture, which often depends heavily on chemical fertilizers and pesticides, RA emphasizes traditional, nature-based practices (6). The five core principles that regenerative agriculture mainly rely to enhance the health of the soil are: minimizing soil disturbance, maximizing crop diversity, maintaining continuous soil cover, keeping living roots in the soil throughout the year, and integrating livestock, such as cattle, into farming systems.
Despite being one of the most effective approaches for sustainable agriculture and atmospheric carbon storage, RA faces several barriers to widespread implementation (7). The approach is time-intensive and demands careful, consistent management. Farmers must adopt new techniques and develop a deeper understanding of soil biology, plant interactions, and ecosystem-based practices to manage their land effectively. These shifts often require both technical training and long-term commitment, which are not feasible for all farming communities.
However, if RA is supported by consistent implementation and integration of modern technologies—such as drones, sensors, and agricultural robotics—it has the potential to become one of the most reliable long-term strategies for carbon storage (8). These technologies can improve the precision and efficiency of land management practices, making it easier to monitor soil health, optimize crop rotation, and reduce labor-intensive tasks. For instance, a study finds that soil has the capacity to sequester up to 3.4 gigatons of carbon per year through agricultural practices (9). Achieving this scale of sequestration, however, would require the planting of approximately 5.72 × 10¹¹ trees annually.
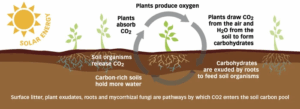
The process of RA involves several interconnected biological and chemical steps that contribute to long-term carbon storage in soils. It begins with the increased cultivation of trees, crops, and plants, which play a critical role in capturing atmospheric carbon dioxide (CO₂) through the process of photosynthesis. During photosynthesis, plants convert CO₂ and water into glucose (C₆H₁₂O₆) and oxygen (O₂), following the reaction: 6 CO₂ + 6 H₂O → C₆H₁₂O₆ + 6 O₂.
The glucose produced is not only used for plant growth but is also exuded through the roots into the soil to support microbial communities. These root exudates stimulate microbial respiration and activity in the rhizosphere, enhancing nutrient cycling. Some of the organic carbon is eventually stabilized through a process called humification, where microbial and plant residues are transformed into complex humic substances. These substances form stable soil organic matter that can remain in the soil for decades, effectively storing carbon in the ground.
Schematic model of photosynthesis. Image from https://www.iasgyan.in/daily-current-affairs/carbon-farming .
Although RA is a highly sustainable approach, meaningful progress through this method requires a simultaneous reduction in anthropogenic carbon emissions. A study indicates that the annual amount of carbon dioxide emitted by human activities exceeds the amount that can be sequestered through regenerative agricultural practices (global annual budget). For example, in 2023, 36.8 gigatons of CO2 were emitted, compared to the 3.4 gigatons of carbon sequestered per year through RA.
Green Concrete: A Surprising Alternative
Mila Kocic
Concrete is a ubiquitous building material; it’s in our roads, our sidewalks, our buildings, our bridges, and our tunnels. In fact, concrete is the second-most utilized substance in the world, second only to water, and “twice as much concrete is used in construction as all other building materials combined” (12). Currently, the world produces 30 billion tons of concrete each year, and global demand for concrete is only increasing, particularly as industrialization accelerates in many countries in the Global South (13). Unfortunately, concrete comes with an energy cost.
Cement, a key component of concrete, is created via a process known as calcination — a mixture of limestone and clay is heated to very high temperatures, which causes a chemical reaction that produces carbon dioxide (CO 2 ) and lime (CaO). The lime is then mixed with more clay and heated again to form cement. Finally, that cement reacts with water to form various hydration products, which harden and bind aggregates (mainly sand, gravel, and crushed rock) together, forming what we know as concrete.
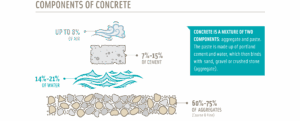
Concrete is made up of four key components: air, water, binder (cement), and coarse and fine aggregates. Image is from https://www.cement.org/cement-concrete/applications-of-cement/ .
However, when the significant energy input required to heat the cement and the CO 2 released as a byproduct during calcination are both taken into account, it becomes evident that concrete has a pretty significant carbon footprint. In fact, one ton of cement releases 0.85 tons of CO 2 , and the concrete industry produces 4-8% of the world’s carbon emissions (14). Currently, no other building material is capable of matching concrete’s versatility, low cost, and ease of production, but is there any way to decrease concrete’s carbon footprint while also potentially cutting costs for the industry?
As it turns out, despite the fact that it emits carbon dioxide during the mixing process, concrete also absorbs carbon through a passive chemical reaction known as weathering carbonation. During this process, calcium hydroxide (Ca(OH) 2 , also known as portlandite), a byproduct of hydration reactions within the concrete, reacts with carbon dioxide in the air to form calcium carbonate (CaCO 3 , also known as calcite). This is a form of carbon sequestration — carbon from the air (CO 2 ) is “sequestered” in mineral form within the molecular structure of the concrete.
However, as a potential strategy for mitigating climate change and decreasing the concrete industry’s carbon footprint, this process has several downsides. The first is simply that it happens very slowly; one ton of concrete absorbs up to 0.9 kg of CO 2 per year via weathering carbonation, although that value is highly dependent on environmental conditions like humidity and temperature (13). This means that the carbon uptake which occurs during weathering carbonation is far less than the carbon emitted by the industry. The second downside is that, under extended exposure to CO 2 , the carbon silicate hydrate gel (C-S-H) that helps bind concrete together decomposes and the concrete begins to degrade. However, we can use the basic chemical reactions which occur during weathering carbonation as a blueprint for designing a technique for sequestering carbon in concrete that is fast-paced and active, as opposed to slow and passive.
As it turns out, the industry currently has two methods. The first is called mineral carbonation, and is essentially a “fast-paced imitation of rock weathering” (13). Mineral carbonation targets binder compounds; normally, cement is simply mixed with water to form the hydration products which bind concrete’s aggregates together. However, if CO 2 is dissolved in water to form carbonic acid (H 2 CO 3 ), hydronium ions from the acid break down the silicate oxides in cement, freeing the cement’s calcium and magnesium ions to form stable carbonates. According to one study, commercial mineral carbonation could sequester up to 3 Gt of carbon per year.
The second method targets the concrete itself. Concrete is often cured after being mixed to ensure rapid hydration reactions, which has beneficial effects for the concrete’s long-term durability and strength. Steam is the typical medium through which concrete is cured, as it allows for high temperature and relative humidity (15), but CO 2 can also be used to the same effect. This process, where CO 2 gas is injected into early-age concrete (i.e., at most a few days after mixing) is known as carbonation curing. Similarly to mineral carbonation, carbonation curing involves the reaction of silicate oxides with water and CO 2 to form CaCO 3 .
Carbonation curing at Michigan State University (video at https://www.youtube.com/watch?v=m6vj0HfSR0Q )
Of course, obtaining pure CO 2 gas, as well as designing and maintaining the closed reaction chambers necessary for curing, means an additional cost for the manufacturer. However, it is possible to cut these costs by substituting the traditional binder and aggregate materials with recycled alternatives to make what is known in the industry as “green concrete.” For example, Portland cement, the typical choice for concrete’s cement component, can be partially or completely replaced with fly ash (a byproduct of the coal industry) or steel slag, since both contain the oxides necessary for hydration reactions to occur. One study found that replacing Portland cement with a mixture of fly ash and lime led to a concrete mixture with a carbonation degree of 78%, compared to a 32% carbonation degree in the Portland cement control mixture (13).
In addition, gravel and crushed rock, the typical choices for coarse aggregates, can be replaced with demolition byproducts (crushed bricks, concrete, etc.). Mineral carbonation can also be performed on these aggregates to increase their strength and durability, as well as their ability to sequester carbon. Finally, fine aggregates (typically sand) can be replaced with biochar, which has the added benefit of being a product of carbon sequestration itself (biochar is formed through burning organic matter at very high temperatures in a low-oxygen environment, leading to the formation of stable carbon structures). Some studies have found that biochar can accelerate hydration reactions during early-age carbonation curing, leading to higher compressive strength (16). Biochar is also highly porous, meaning it has more sites where carbonation reactions can occur.
Unlike CCS or regenerative agriculture, green concrete does not require large-scale change from the existing industry or the creation of a new one altogether; all it takes is incorporating recycled byproducts into the concrete admixture and using carbonation curing technologies instead of steam. Cement manufacturers currently have the means to invest in a more climate-adaptive future — one where we build our cities out of sequestered CO 2 .